Find total number of electrons of the valance shells of carbon and oxygen atoms. The molecular geometry of ClF 3 is T-shaped with asymmetric charge distribution around the central atom.
 Pin On The Art Of Being A Graceful Elegant Lady
Pin On The Art Of Being A Graceful Elegant Lady
IF5 - Iodine Pentafluoride.

Authentic cif3 lewis structure and the description. Drawing the Lewis Structure for BrF 3. Chlorine trifluoride ClF3 CID 24637 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. With an expanded octet of ten electrons a.
O Four bonding pairs of electrons and one nonbonding pair of electrons. Because electrons repel each other electrostatically the most stable arrangement of electron groups ie. Lewis Structures A bond between two atoms is formed by means of sharing of a pair of electrons Each atom shares electrons with neighbors to achieve a total of eight valence electrons Determine connectivity of the atoms in the molecule Sum up the total number of valence electrons in.
F axial-Cl-F axial bond angle. A step-by-step explanation of how to draw the SeF4 Lewis Dot Structure Selenium TetrafluorideFor the SeF4 structure use the periodic table to find the tot. There is a common way by which we can draw the lewis structure of any compound.
O Three bonding pairs of electrons and zero nonbonding pairs of. 32 valence electrons or 16 pair. First draw the Lewis dot structure.
Sp 3 d 2. Iodine Pentafluoride IF5 Molecular Geometry. 22092019 Lewis structure with formal charges Again if you calculate the formal charge for each atom you will notice that chlorine which is the most electronegative element has a positive formal charge.
Apply VSEPR notation A X E ANumber of central atoms XNumber of surrounding atoms E Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3 E 2. You may have heard about the chemical compound that lacks C-H bonds. For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule.
Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions. AX 3 E 2 has T shaped shape. 10 five electron pairs.
Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs. Question 50 1 point Saved Sketch the Lewis structure of CIF3. 08092020 According to this model valence electrons in the Lewis structure form groups which may consist of a single bond a double bond a triple bond a lone pair of electrons or even a single unpaired electron which in the VSEPR model is counted as a lone pair.
CCl4Tally the valence electrons C 1. Therefore ClF3 is polar. 4 4 Cl 4.
Drawing the Lewis Structure for BrF 3. What is the best description of the electrons around the central Cl atom. The S atom has five electron domains around it giving rise to a trigonal -bipyramidal electron -domain geometry.
With C 3 H 6 there are two possible Lewis structures that can be drawn. To simplify the process more for you I. Having a positive formal charge on the most electronegative atom makes the compound unstable and because of this instability structure b is not observed in experiments.
Then draw the 3D molecular structure using VSEPR rules. Boron trifluoride is the inorganic compound and its formula is BF3. Three fluorine atoms contribute.
C is in center with 4. The Lewis structure and electron-domain geometry of SF. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets.
19062021 A lewis structure helps us to find out about the structure of the compound types and the number of bonds physical properties and how the compound interacts with other compounds. The highest repulsion is between any two lone electron pairs resulting in these moving apart as far as possible. Orbital on the sulfur must be used.
03022021 BF3 Lewis Structure Molecular Geometry Hybridization and Polarity. The next highest is between one lone pair and a bond pair. Drawing the Lewis Structure for C 3 H 6.
Are shown in Sample Exercise 92. Such compounds are known as inorganic compounds as they are not the organic ones because of lacking Carbon. Three bonding pairs of electrons and two nonbonding pairs of electrons.
Use lewis structure guidelinesto draw the lewis structure of BrF 5. The lowest is between two bond pairs. Use VSEPR table to find the shape.
Then draw the 3D molecular structure using VSEPR rules. Steps of drawing lewis structure of CO 3 2-Following steps are required to draw the CO 3 2-lewis structure and they are explained in detail in this tutorial. Drawing a lewis structure is pretty simple.
These are arranged in a trigonal bipyramidal shape with a 175. 7 28 Total. Click and drag the molecle to rotate it.
There are a total of 28 valence electrons for the BrF 3 Lewis structure. Chlorine Trifluoride on Wikipedia. In that they full the outer shells of each atom in the structure and use the exact number of valence electrons available for the C3H6 Lewis structure.
Click and drag the molecle to rotate it.
 Vsepr Theory Working Out Shapes Of Molecules Ions Deducing Bond Angles Linear Trigonal Planar Pyramid Bypyramid Tetrahedral Octahedral T Shape Electron Pair Molecular Geometry Beh2 Becl2 Co2 Ag Nh3 2 Bh3 Bf3 Bcl3 Alf3
Vsepr Theory Working Out Shapes Of Molecules Ions Deducing Bond Angles Linear Trigonal Planar Pyramid Bypyramid Tetrahedral Octahedral T Shape Electron Pair Molecular Geometry Beh2 Becl2 Co2 Ag Nh3 2 Bh3 Bf3 Bcl3 Alf3


 Vsepr Theory Working Out Shapes Of Molecules Ions Deducing Bond Angles Linear Trigonal Planar Pyramid Bypyramid Tetrahedral Octahedral T Shape Electron Pair Molecular Geometry Beh2 Becl2 Co2 Ag Nh3 2 Bh3 Bf3 Bcl3 Alf3
Vsepr Theory Working Out Shapes Of Molecules Ions Deducing Bond Angles Linear Trigonal Planar Pyramid Bypyramid Tetrahedral Octahedral T Shape Electron Pair Molecular Geometry Beh2 Becl2 Co2 Ag Nh3 2 Bh3 Bf3 Bcl3 Alf3
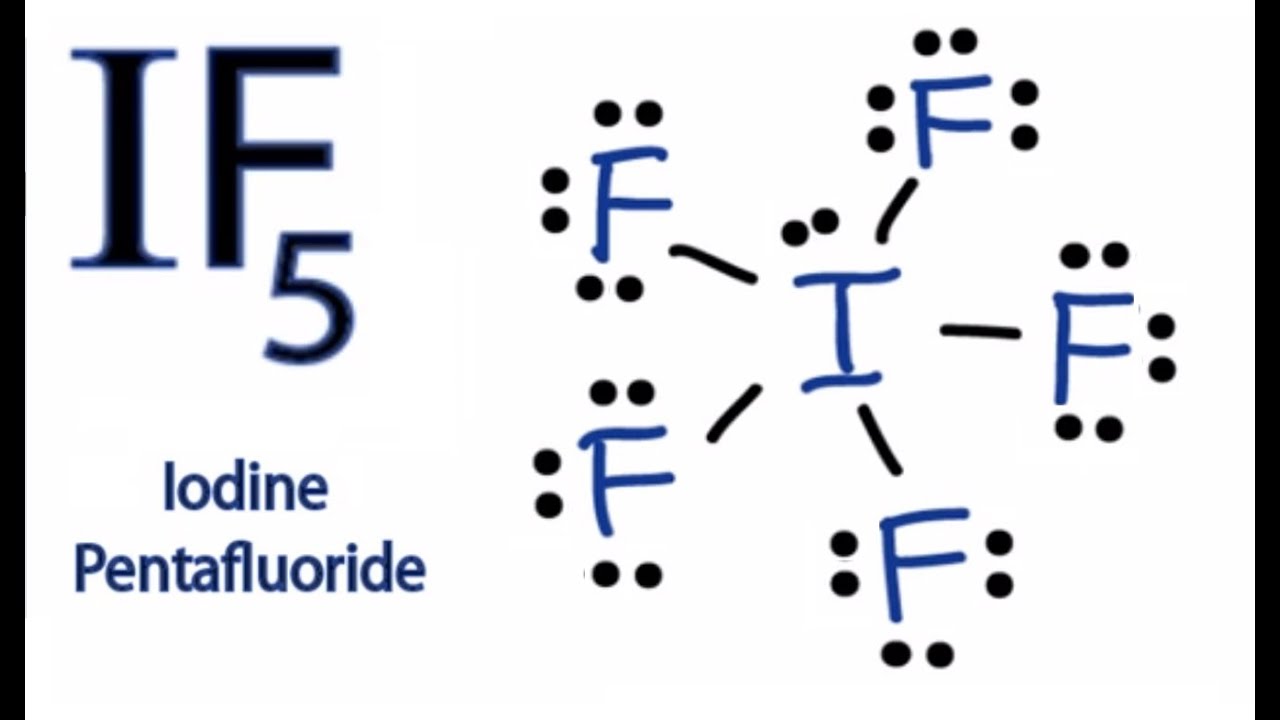 Diagram Lewis Diagram If5 Full Version Hd Quality Diagram If5 Mediagrame Imra It
Diagram Lewis Diagram If5 Full Version Hd Quality Diagram If5 Mediagrame Imra It


 Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo Teaching Science Organic Chemistry Study High School Chemistry
Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo Teaching Science Organic Chemistry Study High School Chemistry
 Learn About Chemistry S Octet Rule Electrons And Element Stability Chemie
Learn About Chemistry S Octet Rule Electrons And Element Stability Chemie
 Diagram Lewis Dot Diagram For Cl Full Version Hd Quality For Cl Diagramaperu Mariachiaragadda It
Diagram Lewis Dot Diagram For Cl Full Version Hd Quality For Cl Diagramaperu Mariachiaragadda It
 Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Dots Drawings Lewis
Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Dots Drawings Lewis
 I Made The Lewis Dot Structure On Cookies For A Chemistry Project The Class Loved Them Chemistry Projects Chemistry Teaching Science
I Made The Lewis Dot Structure On Cookies For A Chemistry Project The Class Loved Them Chemistry Projects Chemistry Teaching Science
 Another Word For Because What Is Another Synonym Word For Because Every Language Spoken Around The World Has Essay Writing Skills Learn A New Language Words
Another Word For Because What Is Another Synonym Word For Because Every Language Spoken Around The World Has Essay Writing Skills Learn A New Language Words
