The mechanism for the reaction between ethene and a molecule X-Y. A The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction.
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Equilibrium equations or Equations of Motion in cylindrical.

Authentic reaction coordinate diagram and the description. Reaction coordinate diagram of acetolysis of anti-7-norbornenyl tosylate and exo-2-bicyclohept-6-enyl tosylatepng 1200. 07052021 Longitude is measured 180. Choose the best description of an enzyme.
Catalyzed reactions have a lower activation energy rate-limiting free energy of activation than the corresponding uncatalyzed reaction resulting in a higher reaction rate at the same temperature. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. 01122020 Label ΔH as positive or negative.
0 illustrate the change in the potential energy of the system as reactants are converted to products. 13122019 The potential energy diagrams for a reaction with a ΔE. 42 A free energy G diagram for a simple reversible exothermic reaction APsolid and broken lines.
Figure shows the energy level diagram for the reaction between methane and oxygen. It is very unlikely that any two different atoms joined together will have the same electronegativity. The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction.
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. 23092018 The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. G A G P Initial state Final state Fig.
B It increases the rate at which a chemical reaction approaches equilibrium relative to its uncatalyzed rate. This first video takes you through all the basic parts of the PE diagram. Click card to see definition.
The following 19 files are in this category out of 19 total. This is a somewhat complicated measure of how far the reaction has progressed or how much of the reaction has happened. G A and G P represent the average free energies per mole for the reactant A and the product P the initial and.
03112006 Gibbs free energy reaction coordinate profiles found in some textbooks. Label the energy diagram and answer the question that follows1. However the path may be more complex or the problem may have other attributes that make it desirable to use cylindrical coordinates.
16012015 Chemical Kinetics Reaction Coordinate Diagrams It is helpful to visualize energy changes throughout a process on a reaction coordinate diagram like this one for the rearrangement of methyl isonitrile. CYLINDRICAL COORDINATES Section 136 This approach to solving problems has some external similarity to the normal. For a reaction such as the one shown in b E a must be greater than ΔE.
Hydrogenation of a double bond is a thermodynamically favorable reaction. 0 and b ΔE. Note that the x axis is officially titled reaction coordinate.
Tangential method just studied. The overall change in energy in a reaction is the difference between the energy of the reactants and products. It is often more convenient to assume the reaction is going.
Sometimes a teacher finds it necessary to ask questions about PE diagrams. C It makes a reaction thermodynamically favorable. Media in category Reaction coordinate diagrams.
As aids to locate longitudinal positions on a globe or map meridians are plotted and drawn from pole to pole where they meet. At a somewhat constant rate and think of the x axis simply as time. Reaction coordinate diagrams are derived from the corresponding potential energy surface PES.
This restriction may be circumvented by the use of a catalyst as shown in the reaction coordinate diagram below. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. B During the reaction the temperature of the mixture increases.
In a hydrogenation reaction two hydrogen atoms are added across the double bond of an alkene resulting in a saturated alkane. The distance per degree of longitude at the Equator is about 11132 km 6918 miles and at the poles 0. Both east and west of the prime meridian.
Based on Figure the following information can be obtained. Chemical Kinetics Reaction Coordinate Diagrams It shows the energy of the reactants and products and therefore E. Energy profile chemistry For a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single energetic pathway along the reaction coordinate as the reactants are transformed into products.
The line increases from the Reactants region to three peaks and two troughs before decreasing to the lowest potential energy level at the Produ region Potential energy im Reaction coordinate. Reaction coordinate Transition state ΔG 1 ΔG 1 ΔG 1 ΔG 1 ΔG. A It allows a chemical reaction to proceed extremely fast.
In both cases E_a is positive. An example of an alkene addition reaction is a process called hydrogenation. We are going to assume that Y is more electronegative than X so that the pair of electrons is pulled slightly towards the Y.
Reaction Coordinate Diagram of.
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
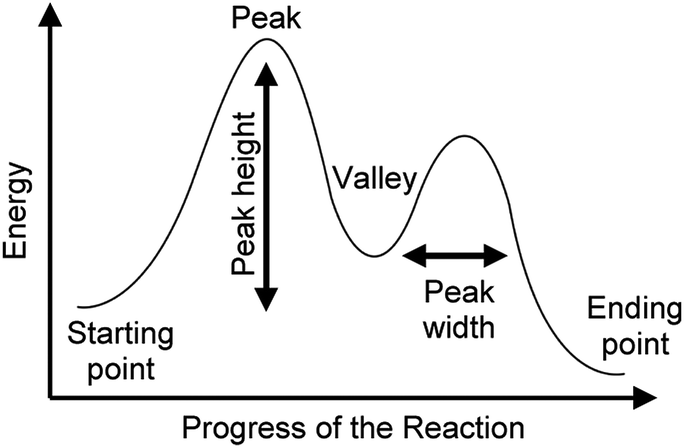 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
 Two Reaction Coordinate Diagrams Graph A With A Free Energy Of Its Products Lower Than The Free Energy Of Its Subst Energy Activities Free Energy Coordinates
Two Reaction Coordinate Diagrams Graph A With A Free Energy Of Its Products Lower Than The Free Energy Of Its Subst Energy Activities Free Energy Coordinates
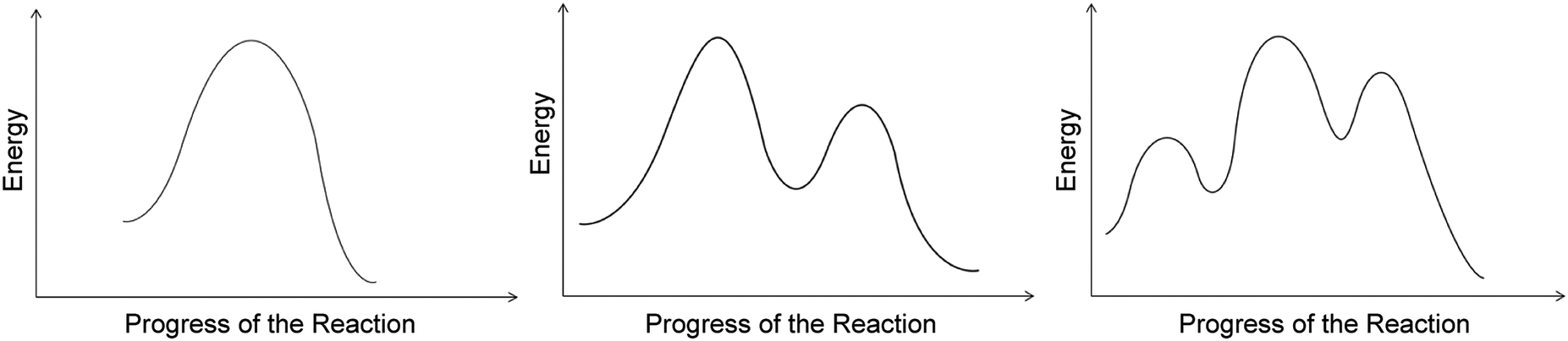 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
 Quantum Mechanical Tunneling Is Essential To Understanding Chemical Reactivity Trends In Chemistry
Quantum Mechanical Tunneling Is Essential To Understanding Chemical Reactivity Trends In Chemistry
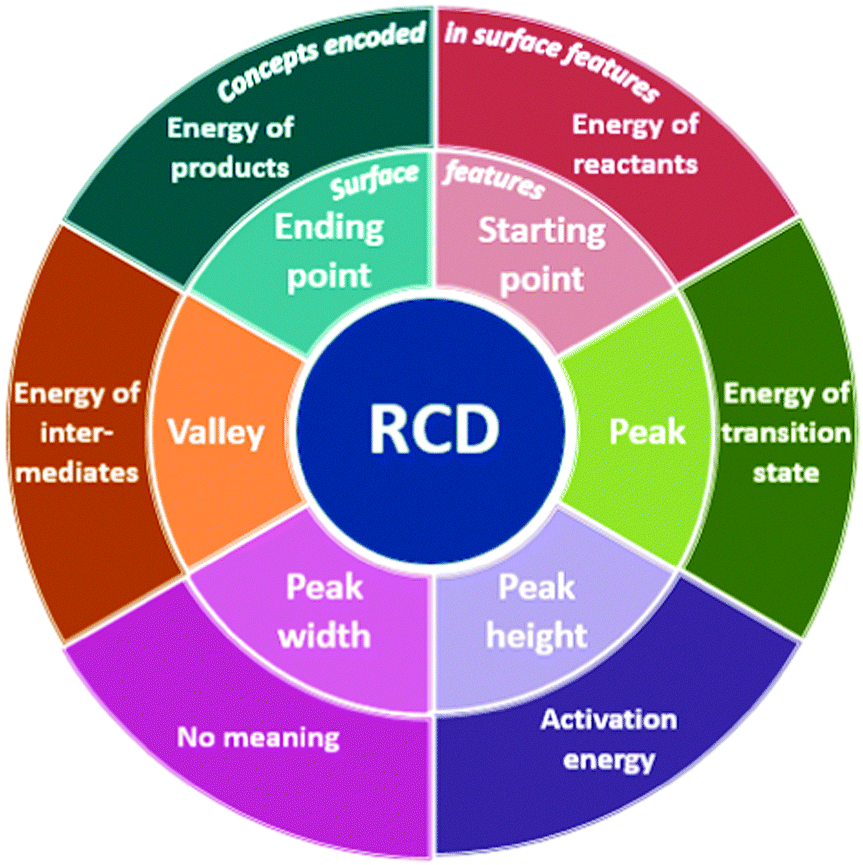 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
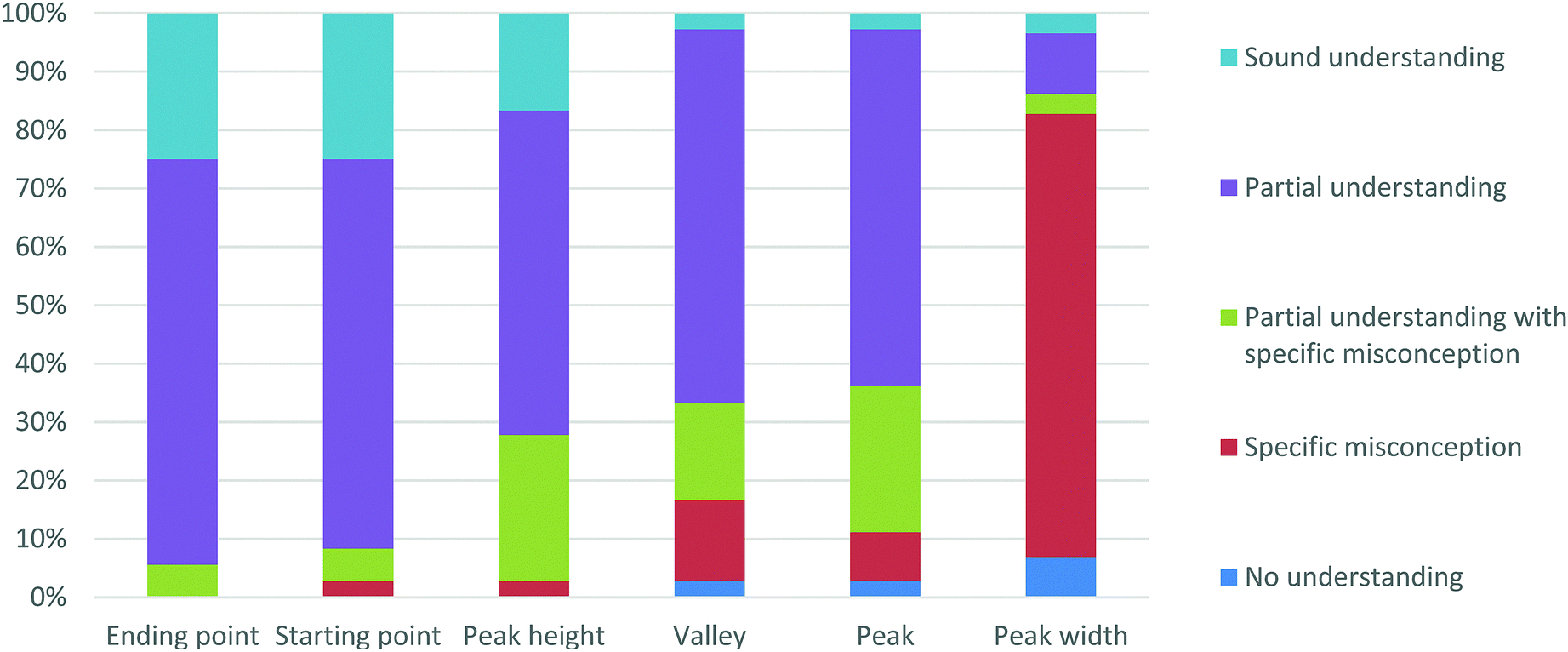 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
 Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
Organic Chemistry Students Challenges With Coherence Formation Between Reactions And Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00064f
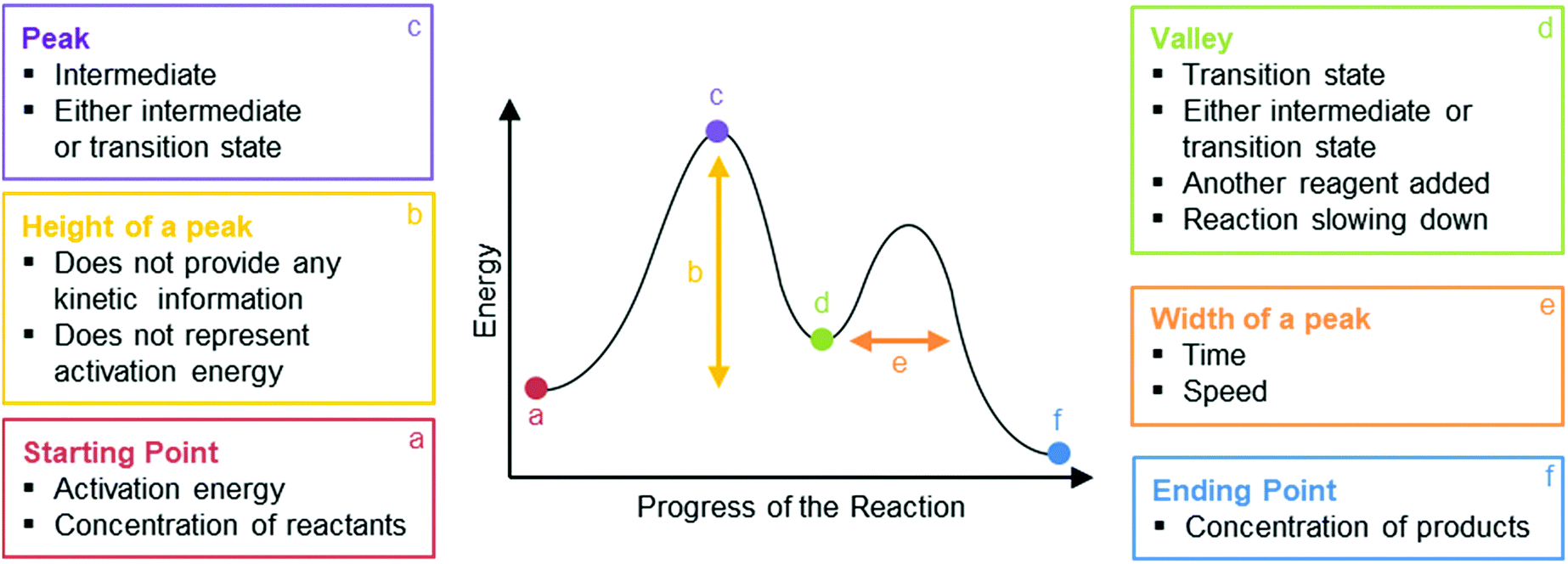 Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h
Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Chemistry Education Research And Practice Rsc Publishing Doi 10 1039 C8rp00063h